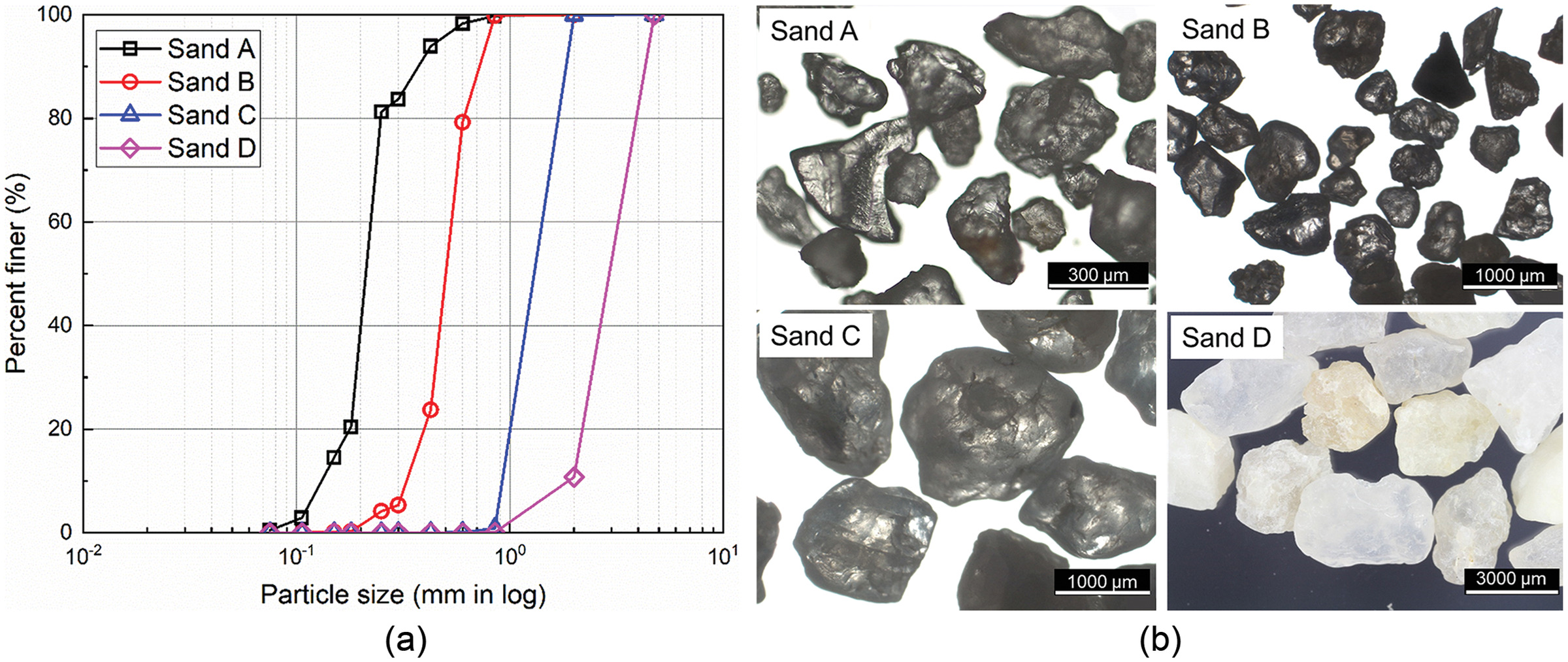
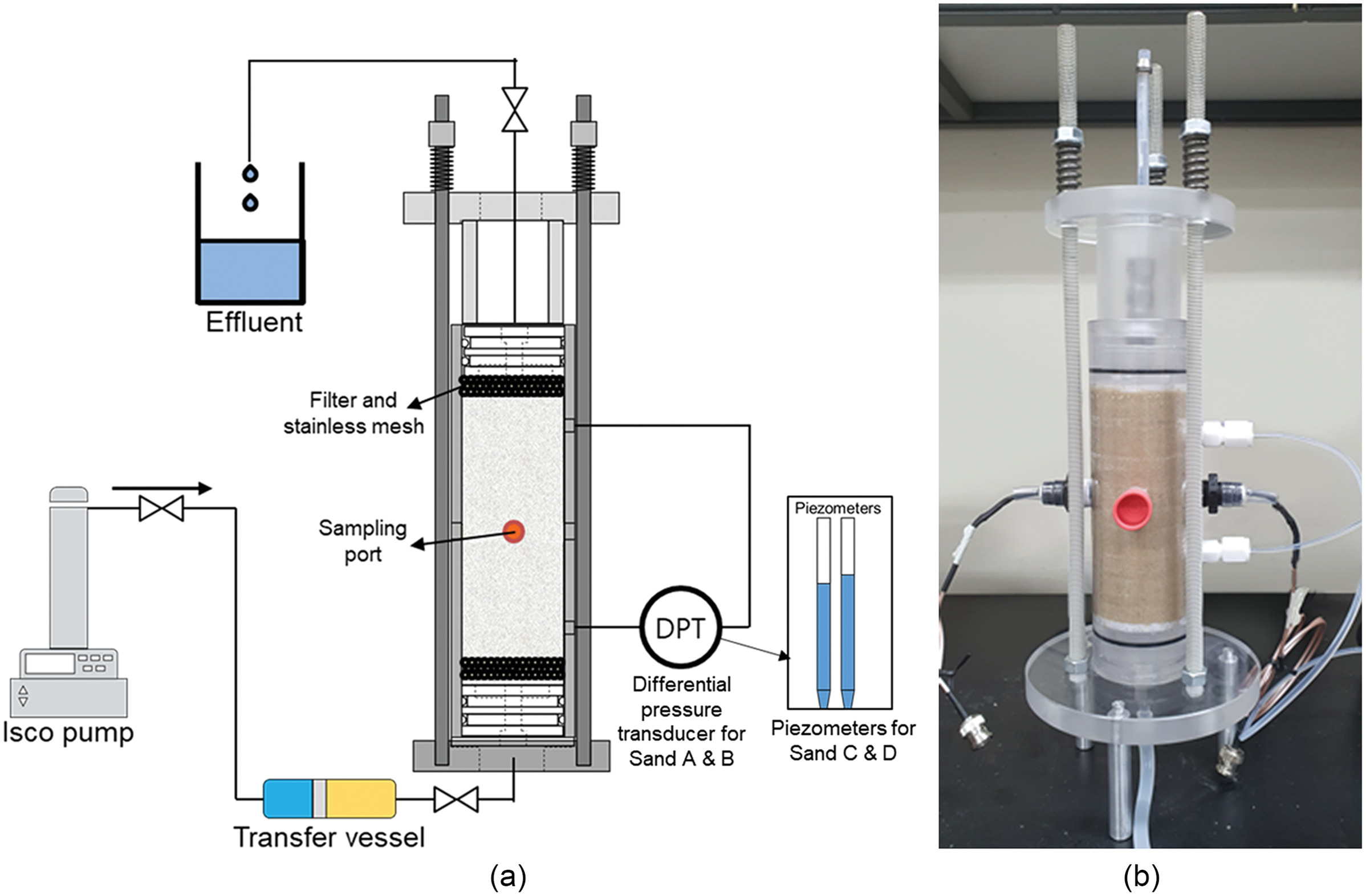
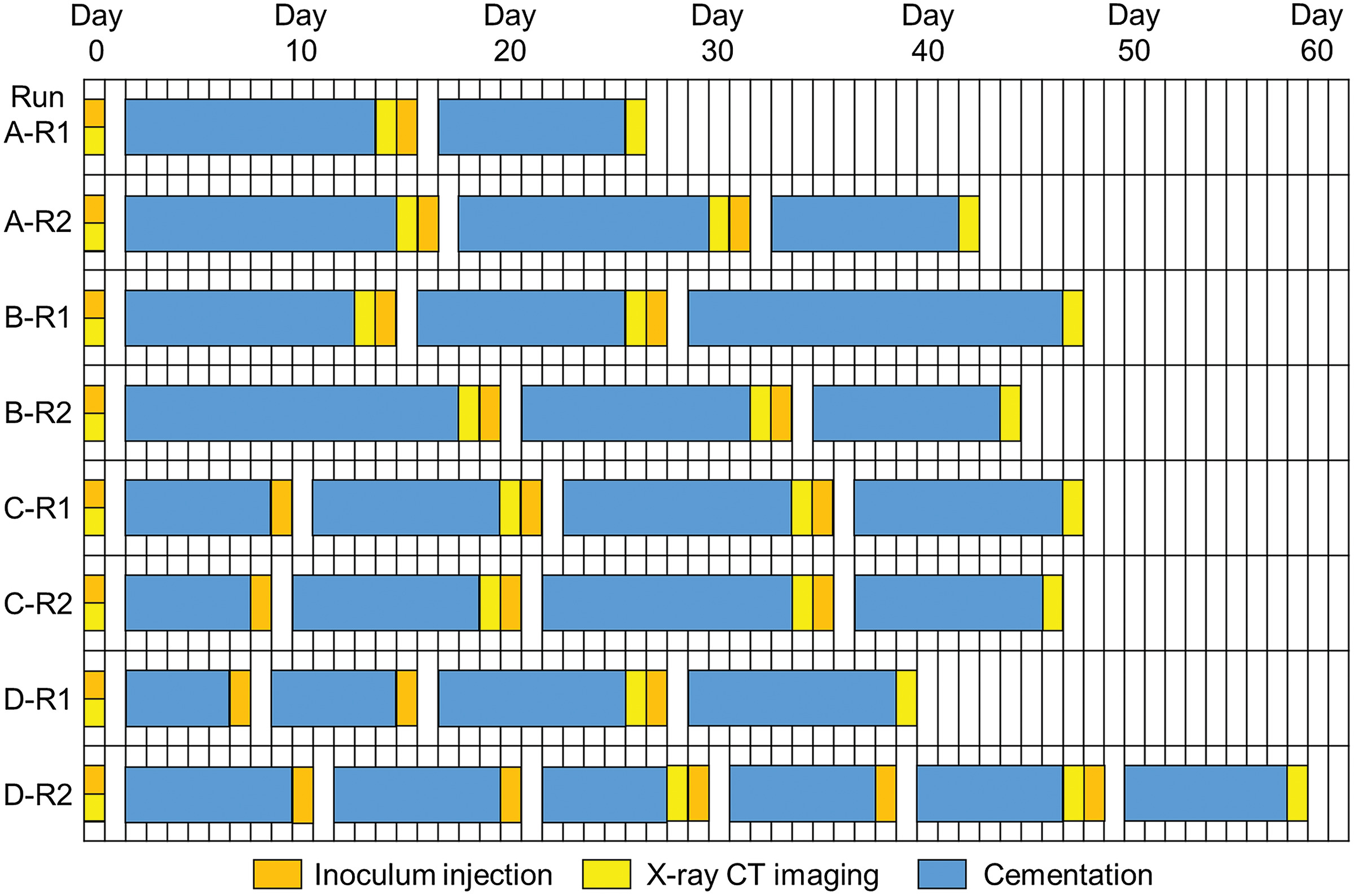
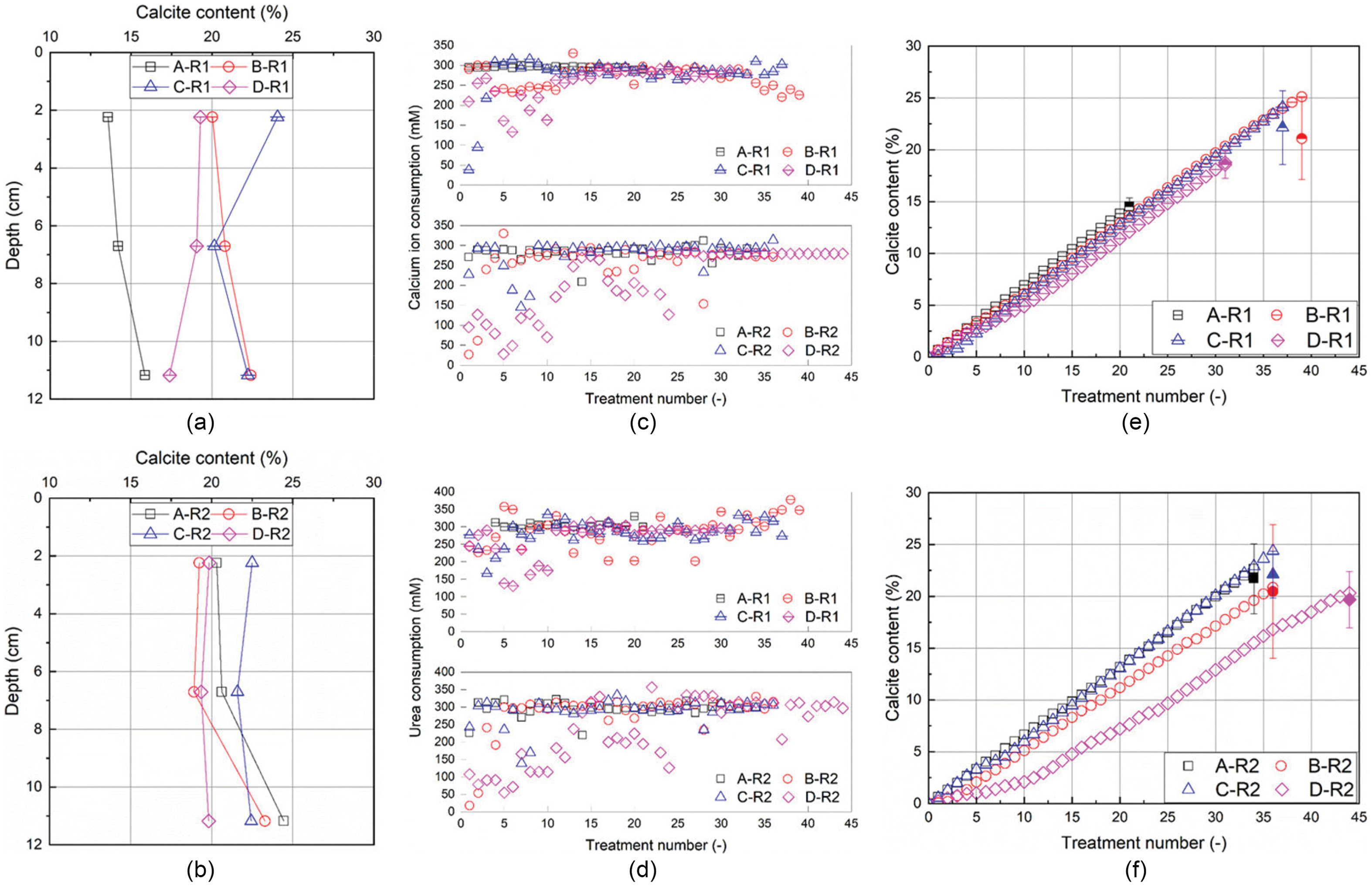
Generalized Kozeny–Carman Model
The Kozeny–Carman (KC) model is widely used to capture the effect of porosity reduction on permeability,
, and hydraulic conductivity,
. A basic formulation of the KC model can be expressed for hydraulic conductivity,
, as follows (
Carman 1937;
Kozeny 1927;
Scheidegger 1958):
where
= absolute permeability;
= fluid density;
= gravitational acceleration;
= fluid viscosity;
= shape factor;
= porosity;
= geometric tortuosity; and
= specific surface area per sediment bulk volume. The KC model can be further generalized by substituting the power exponent to
and by convoluting the changes in the geometric tortuosity and specific surface area in a single shape factor term. Thereby, the normalized hydraulic conductivity,
, can be expressed as a function of the normalized porosity,
, as follows:
where the subscript
indicates the initial untreated baseline condition. The comparison shown in Fig.
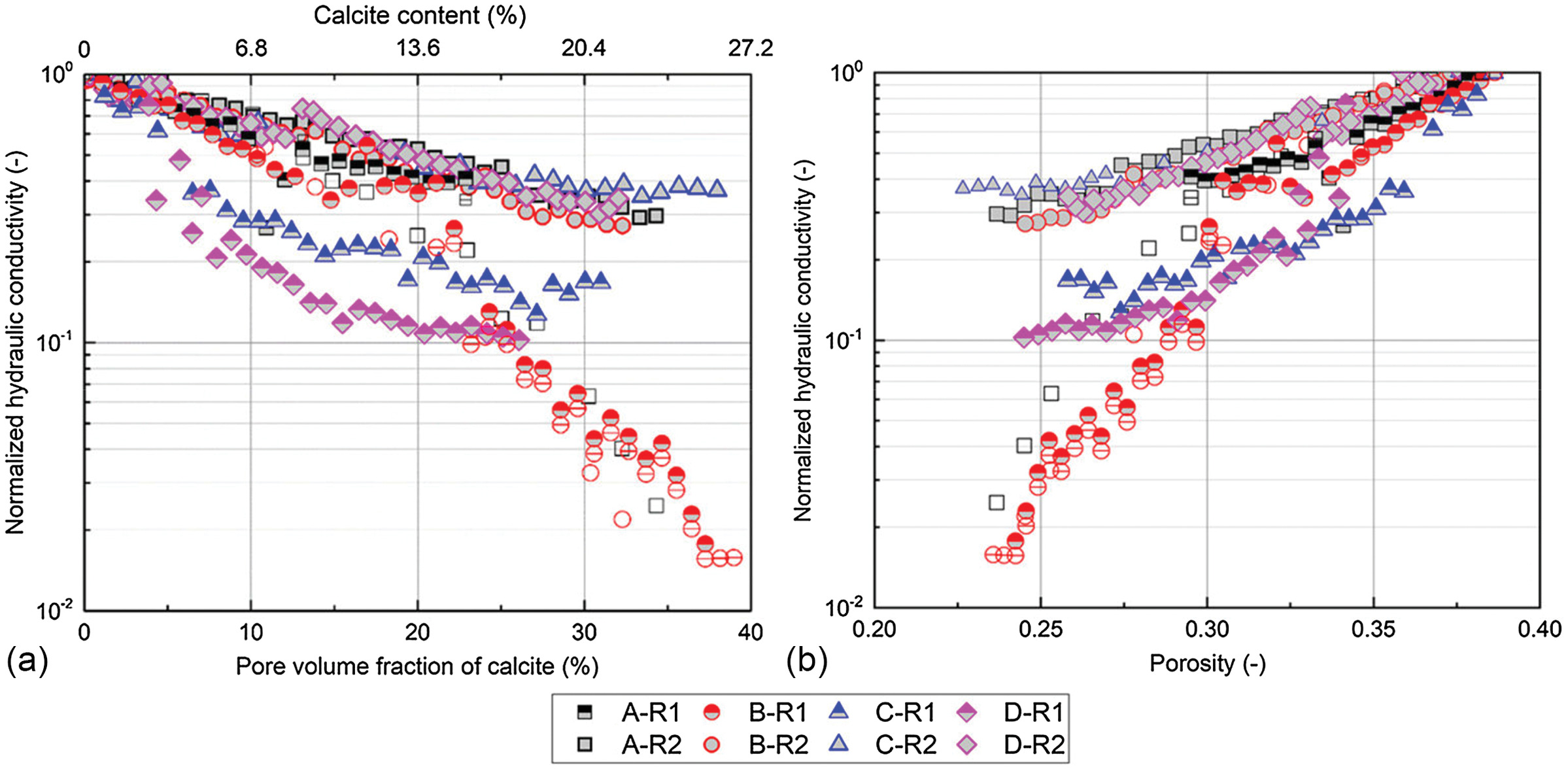
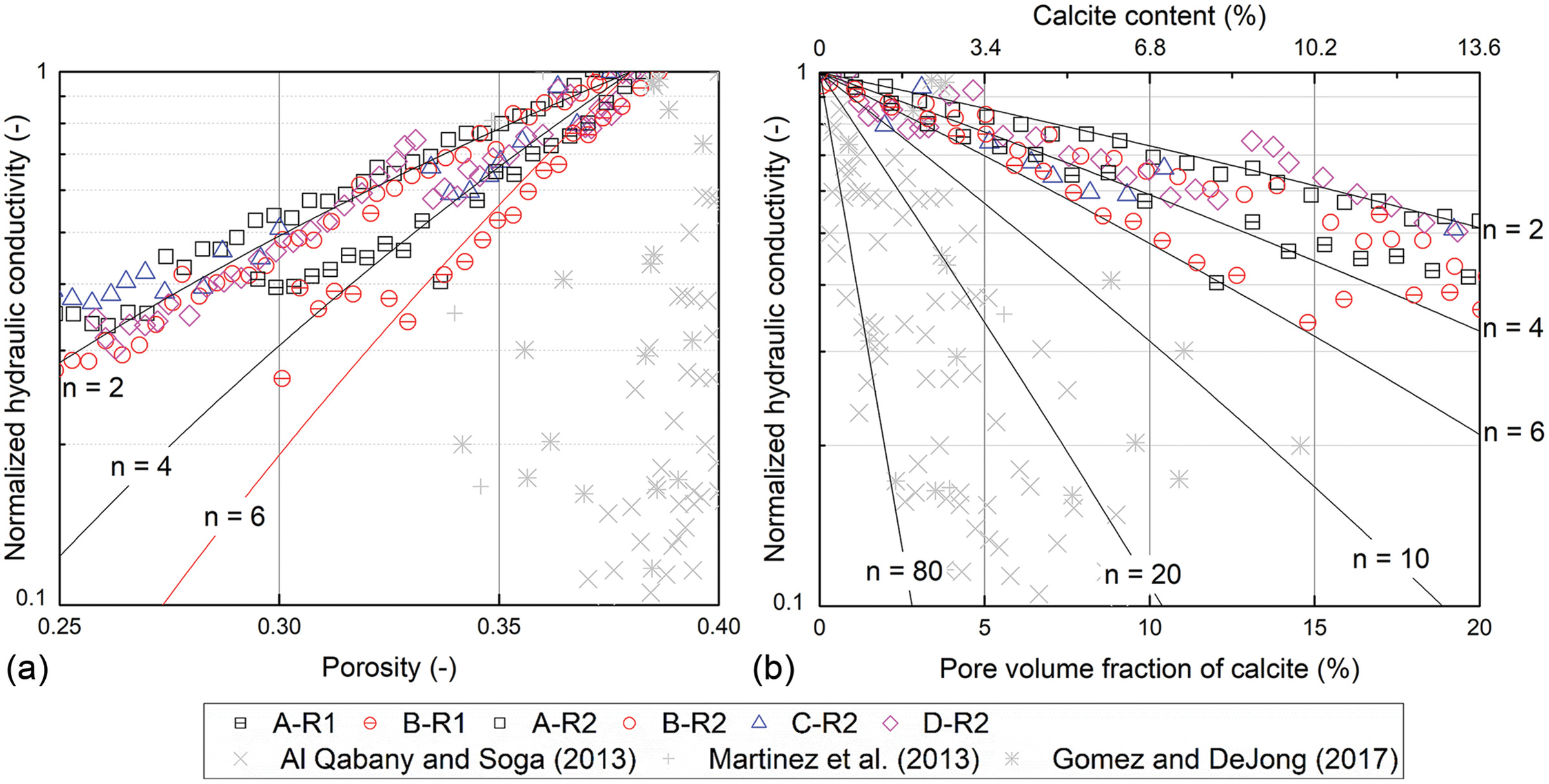
13(a) reveals that the generalized KC model can predict the measured
reduction trends with an exponent,
, of 2–6.
Kozeny Grain-Coating Model
A Kozeny grain-coating model is a model derived from the Kozeny model by assuming that the mineral precipitation uniformly coats soil particles (
Kleinberg et al. 2003;
Noh et al. 2016;
Baek et al. 2019). The Kozeny grain-coating model expresses the normalized hydraulic conductivity (
) as the pore volume fraction of calcite (or calcite pore saturation):
. The detailed derivation is described in the Appendix. As shown in Fig.
13(b), the exponent,
, contributes to the decrease in hydraulic conductivity, and as
increases, the hydraulic conductivity reduction accelerates. The term (
) is identical to
; therefore, this model is equivalent to the generalized Kozeny–Carman model [Eq. (
2)]. Accordingly, the Kozeny grain-coating model with an exponent
for
also shows good agreement with the measured
data in this study. Meanwhile, the Kozeny grain-coating model requires an
value of 10–80 to fit the previously published data [Fig.
13(b)].
However, at higher cementation levels, such as
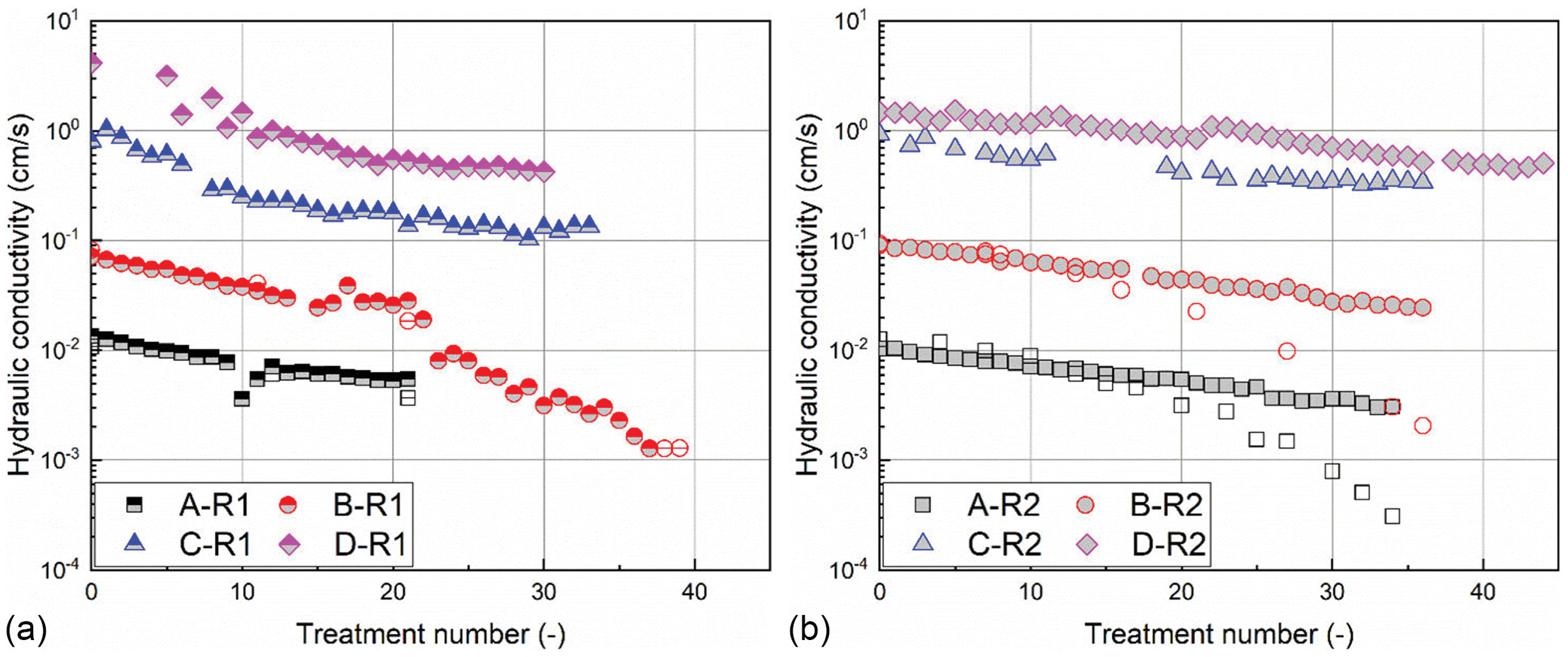
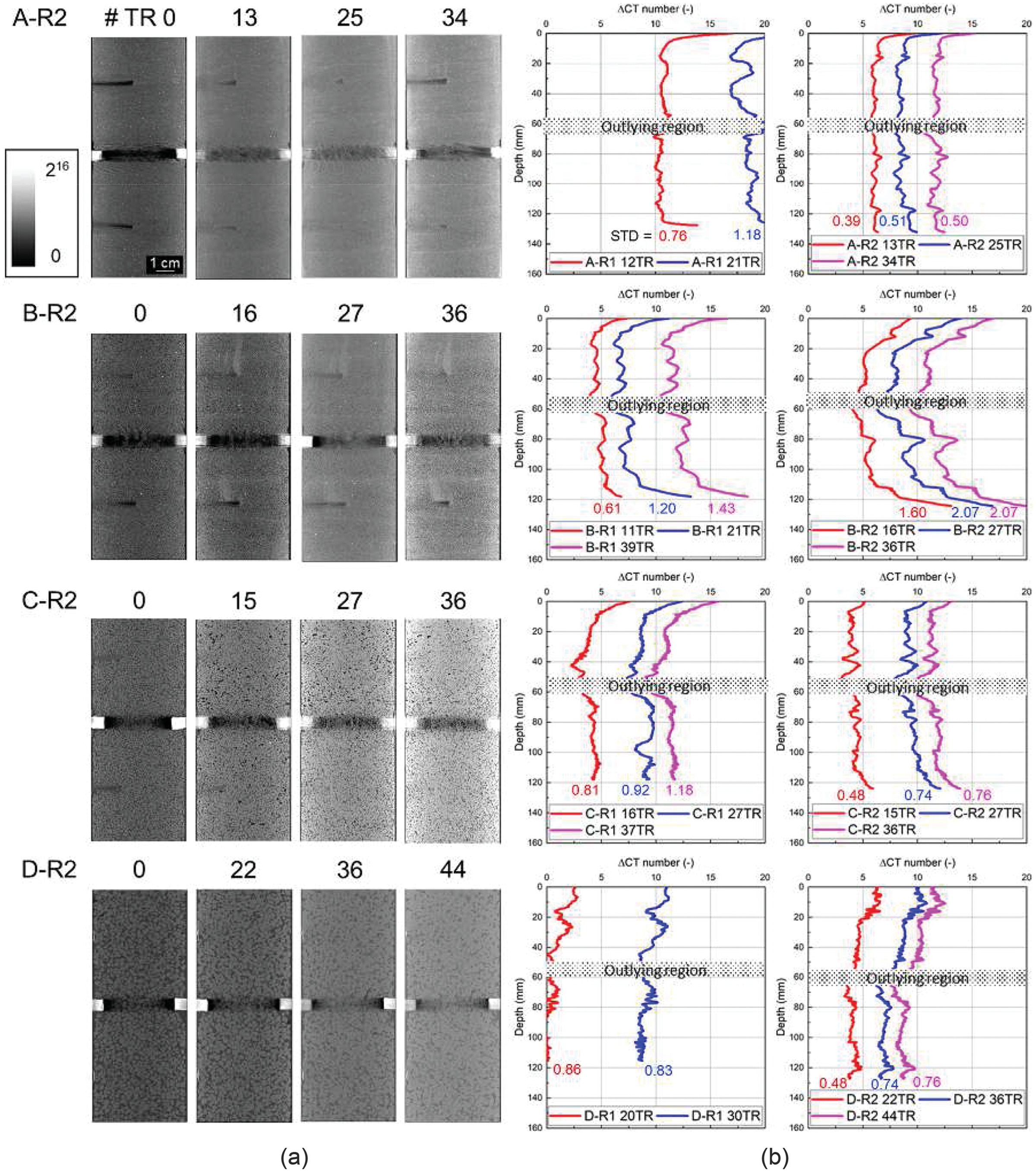
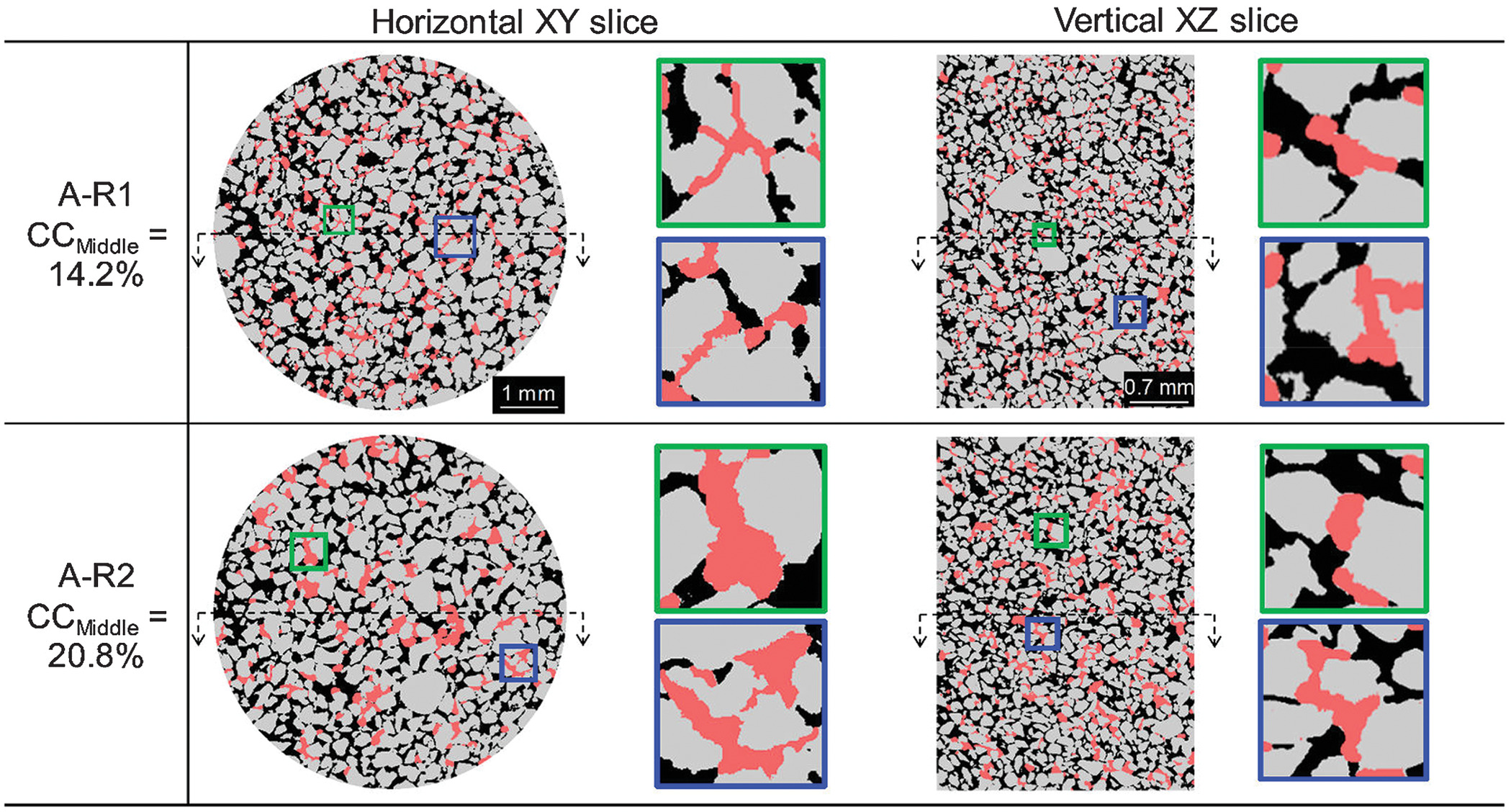
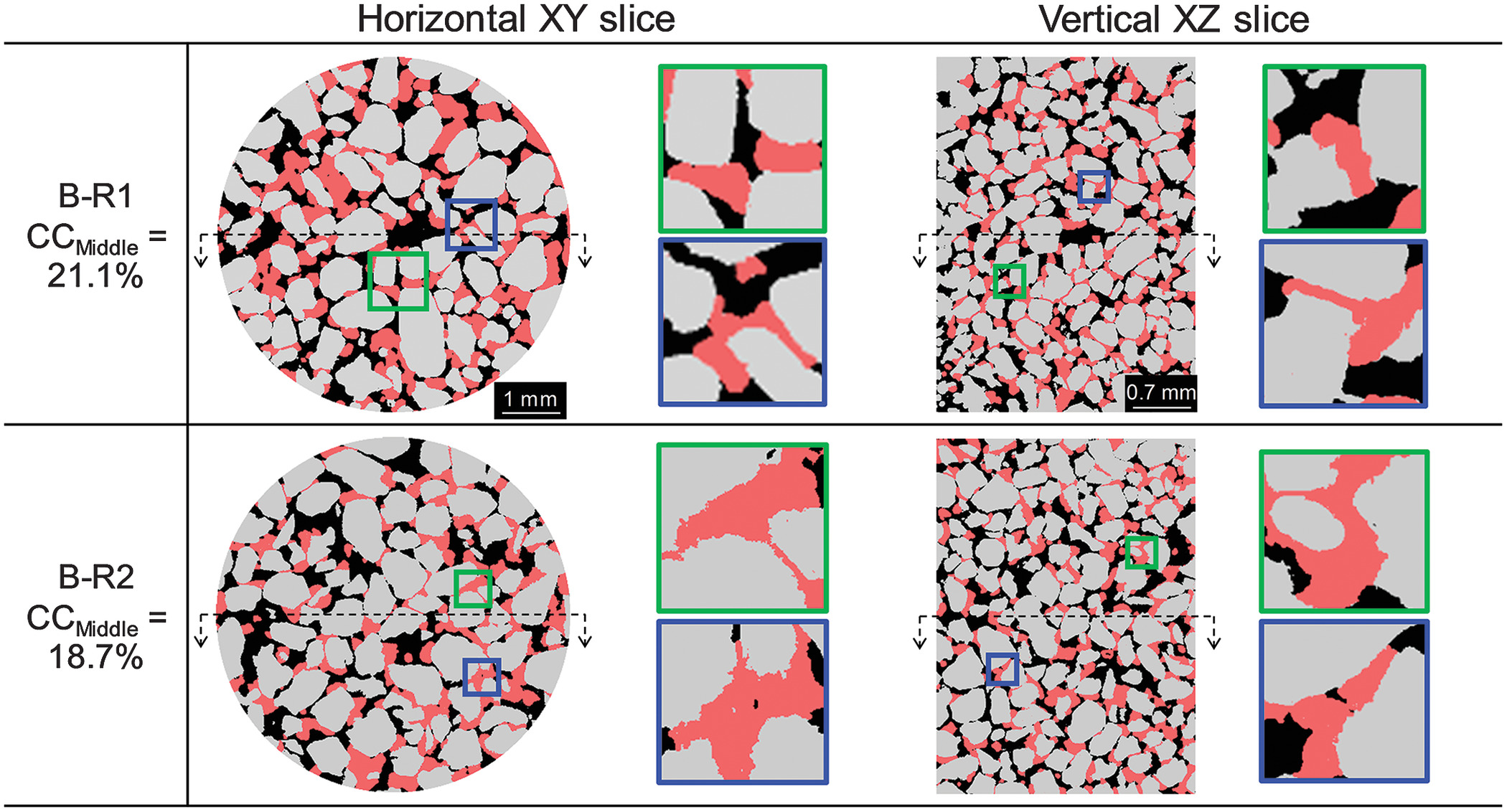
, a local blockage becomes more probable, which results in a drastic reduction in hydraulic conductivity [see B-R1 in Fig.
5(a) as an example]. This is consistent with Baek et al. (
2019), in which abiotic precipitation of
from supersaturated solutions exhibited a uniform grain-coating behavior and a sharp reduction in
due to clogging when
. In their study, such a clogging-driven
reduction rate corresponded to an
for the Kozeny grain-coating model while
showed a gradual decrease when
with
(
Baek et al. 2019).
Other Hydraulic Conductivity Reduction Models
The Dai–Seol model, a derivative of the Kozeny–Carman equation, is based on the statistical results from a number of pore network modeling studies of tortuous flows, in which crystal precipitation is randomly generated (
Dai and Seol 2014). The model is defined as
where
= fitting parameter and ranges from 0.1 to 4. With the relationship between the relative porosity
and
as
and Eq. (
2), the normalized hydraulic conductivity,
, is expressed as a function of pore volume fraction of inclusion material, calcite (
) in this case, as follows:
where
= fitting parameter, which ranges from 0.1 to 4, and the variables with subscript
denote the values at a given
. The relative porosity,
, is defined as
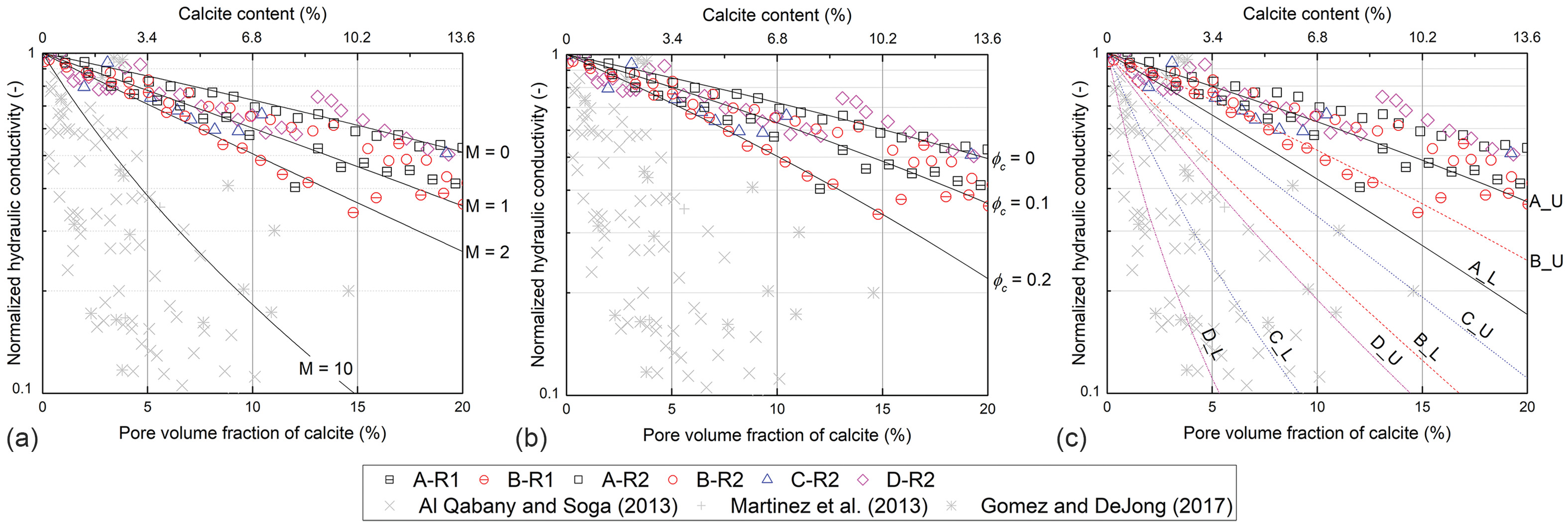
. Fig.
14(a) shows that the Dai–Seol model with
from 0 to 2 predicts the measured
reduction trends in this study, which is consistent with the typical range of tortuosity and specific surface area. On the other hand, fitting the model to previously published data requires the
value to be greater than 10.
The second model is the Kozeny–Carman-based effective porosity model (
Wang and Nackenhorst 2020). This model introduces the concept of the effective porosity,
, defined as the volume fraction of pore that is still connected to each other after calcite precipitation, and the critical porosity,
, defined as the porosity at which the permeability becomes zero. Koponen et al. (
1997) expressed the effective porosity, as follows:
where
is a constant;
is defined as
; and
is the total porosity. The characteristics of pore structures determine
and
. Considering the effective porosity in the modified Kozeny–Carman model, Eq. (
1) becomes
where the subscript
indicates the initial value. Fig.
14(b) shows the predicted
reductions while varying the critical porosity
. The critical porosity
controls the rate of
reduction for a given porosity reduction; the higher
indicates a faster rate of
reduction. The measured
mostly falls into the bounded region with
and 0.2. Meanwhile, fitting of the effective porosity model to previously published data requires
, which seems not physically plausible in soils.
Finally, the Panda–Lake model (
Panda and Lake 1994), which is also a derivative of the Kozeny–Carman model, introduces three reduction factors associated with mineral precipitation: porosity reduction factor (
), tortuosity reduction factor (
), and specific surface area reduction factor (
). These three factors are determined based on the morphological characteristics of calcite precipitate distribution, including pore-lining, pore-filling, and pore-bridging behavior, respectively. The most relevant model to the observed pore-scale MICP pattern is the pore-lining behavior. Among the three factors,
is assumed to be 1, owing to its minimal effect on pore structure (
Panda and Lake 1994). Therefore, the Panda–Lake equation can be rewritten as follows:
where the subscript
indicates the initial value;
is the specific surface area of host soil; and
is the specific surface area of
minerals. As the host particle size decreases and the specific surface area of the particle
increases, the rate of
reduction becomes faster. And, as the size of
precipitates decreases and the specific surface area of
increases, the rate of
reduction becomes faster.
can be calculated assuming a spherical particle with a mean grain size. Therefore, the important and tunable variable is only the specific surface area of
minerals,
, in this model. The lower and upper limits of
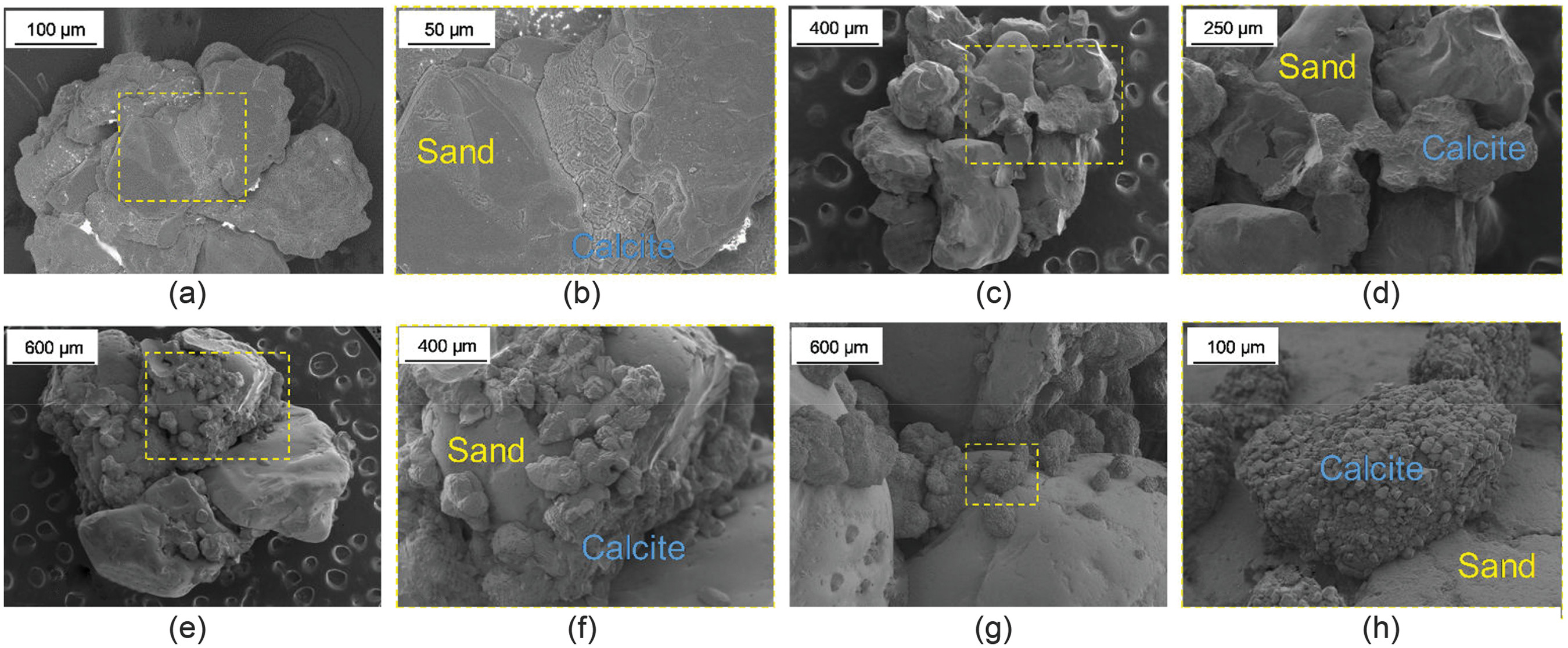
were estimated based on SEM images, assuming a spherical crystal sized as 50 and 200 μm. As a result, there are two bounding
reduction curves for each soil, as shown in Fig.
14(c). The Panda–Lake model predicts a wide variation in
reduction trends with the host grain size; however, it appears that the modeling results do not match well with the experimental results of this study.